Help on OWL Problem Ch 2 - Question 12
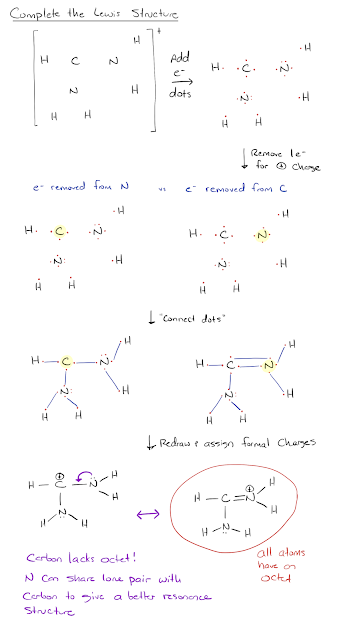
Many of you have had trouble with one particular part of this question from OWL. The issue that most of you are encountering is you end up with a Lewis structure where carbon has a +1 formal charge. Sometimes this is unavoidable. In this problem, however, since there is a nitrogen (with a lone pair) next to the carbon, it can donate its lone pair through resonance to give a better structure where all atoms have an octet. See the attached solution below.
Comments
Post a Comment